 7Photo© news18.com
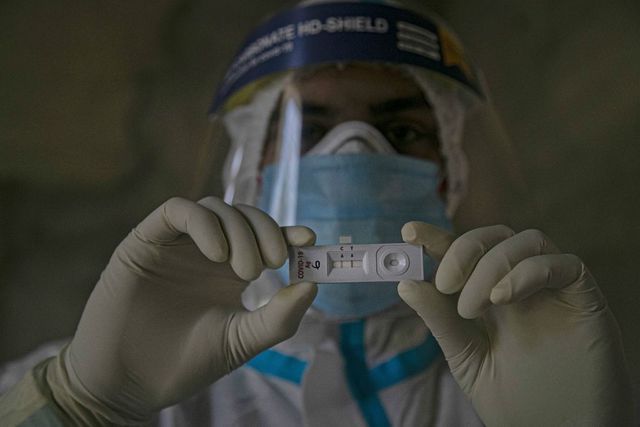
7Photo© news18.comIndia’s first CRISPR Covid-19 test approved for use
, 19 news, a view
The Tata CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) test, powered by CSIR-IGIB (Institute of Genomics and Integrative Biology), received regulatory approval on Saturday from the Drug Controller General of India for its commercial launch, meeting high-quality benchmark with 96 per cent sensitivity and 98 per cent specificity for detecting novel coronavirus.
The test uses an indigenously developed, cutting-edge CRISPR technology for detection of the genomic sequence of the SARS-CoV-2 virus. CRISPR is a genome editing technology for diagnosing diseases.
The Tata CRISPR test is the world's first diagnostic test to deploy a specially adapted Cas9 protein to successfully detect the virus causing Covid-19.